Abstract
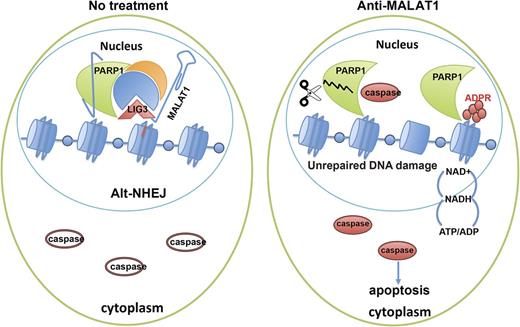
Molecular pathogenesis studies of multiple myeloma (MM) have focused on coding genes rather than long non-coding RNAs (lncRNAs). However, evidence indicates that lncRNAs are involved in the initiation and progression of almost all kinds of cancers, including MM. MALAT1 (metastasis-associated lung adenocarcinoma transcript 1) is one of the most highly expressed conserved nuclear lncRNAs. Here, we demonstrated that MALAT1 plays important roles in MM DNA repair and cell death. We found that bone marrow plasma cells from patients with MGUS and MM express elevated levels of MALAT1 relative to those from healthy individuals. Our functional studies suggested that in MM, MALAT1 is key in regulating the DNA repair alternative non-homologous end-joining (A-NHEJ) and apoptosis pathways. Using RNA Antisense Purification-Mass Spectrum (RAP-MS), we identified MALAT1 binding proteins in MM, including PARP1 and LIG3, two key components of the A-NHEJ pathway protein complex. Degradation of the MALAT1 RNA by RNAse H using antisense gapmer DNA oligos in MM cells induced poly-ADP-ribosylation of nuclear proteins, defected the DNA repair pathway, and further induced apoptotic pathways. Anti-MALAT1 therapy combined with PARP1 inhibitor or proteasome inhibitor in MM cells showed a synergistic effect in vitro. Furthermore, using a novel single wall carbon nanotube (SWCN) conjugated with anti-MALAT1 oligos, we successfully knocked down MALAT1 RNA in cultured MM cell lines and in two xenograft mouse models. Most importantly, anti-MALAT1 therapy also could induce DNA damage and cell apoptosis in vivo, indicating that MALAT1 could serve as a potential novel therapeutic target for MM treatment.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal